COVID-19 Antibody Testing Guide
Rhino Summary
This guide is based upon the CDC’s Interim Guidelines for Antibody Testing, which can be found here: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html, recently published clinical studies, and information available from Center for Medicare and Medicaid Services (CMS).
Coronavirus and Antibodies
Antibodies will play an important role in monitoring and responding to COVID-19.
Antibodies usually become detectable 1-3 weeks after infection.
Infectiousness greatly decreases after antibodies develop.
Antibodies give people immunity, but how strong the immunity is and how long it lasts requires further clinical studies.
Because of these unknowns, antibody testing should not be used to make decisions about public health, including social distancing and use of PPE … yet.
However, preliminary and ongoing clinical studies are promising, showing strong and long-lasting immunity.
How to Perform Antibody Testing
Use tests with the highest sensitivity & specificity possible, 90-95% or higher.
Test individuals with a high likelihood of infection, especially those with prior symptoms.
Use two different tests to increase predictive value by 30% or more in many cases (orthogonal testing).
Use antibody tests to supplement PCR tests, especially after the first week of symptoms.
Test Cost, Medicare and Insurance Reimbursement
We have two FDA EUA authorized antibody tests available.
The CMS reimbursement rate for our antibody tests is $45.23 per test. Private insurance reimbursement rates may be higher.
The CDC recommends performing two different tests per patient to improve accuracy.
Coronavirus and Antibodies
Antibodies usually develop within 1-20 days after symptoms appear during a viral infection. The most important antibodies for COVID-19 are IgM and IgG because they are found in the highest concentrations in blood. Most coronavirus antibody tests detect IgM, IgG, or both.
In the vast majority of infections, development of antibodies renders a person immune to future infection. It also minimizes the probability of transmitting an infection to another person. However, coronavirus is new and there are no long-term studies to confirm how strong immunity is, how long it lasts, or the chances of infecting another person once immune.
Published studies have shown that the immune response in coronavirus patients is a bit unusual. Typically, IgM appears first and IgG appears second. Then IgM disappears over a few weeks, and IgG stays around for weeks, months, or years to prevent re-infection. But in coronavirus patients, IgM and IgG have been shown to appear around the same time, on average.
According to a recently published study, the median time it took to develop antibodies was 13 days. One hundred percent of patients developed antibodies by Day 19.
Existing studies have shown that recurrence of coronavirus infection is very rare, which suggests that antibodies give strong immunity and greatly reduce the likelihood of infecting another person.
However, the CDC cautions that since coronavirus is new and long-term studies have not yet been completed, individuals, physicians, and organizations should remain cautious and not assume immunity or make decisions based upon an antibody test result until further studies are published.
Antibody testing should be performed:
To Supplement Diagnostic Testing. Antibody testing should be used to supplement diagnostic testing. Diagnostic testing (PCR) is most accurate during the first week of infection and becomes less reliable thereafter. A recent study showed that using antibody testing to supplement PCR testing increased the accuracy of diagnoses during all stages of infection. If a physician is concerned a diagnostic test gave a false result, they should consider using an antibody test to supplement diagnosis. Antibody tests become more sensitive than diagnostic tests after around Day 8 of infection.
To Determine Prevalence of COVID-19 in a Population. Because antibodies to coronavirus remain detectable in blood for months after infection, antibody testing is the best method to determine the percentage of a population previously infected. If a person tests positive for antibodies, it indicates he or she was previously infected and now has some level of immunity. When around 50% or more of a population has been infected, the population develops herd immunity, which means the risk of spread is greatly reduced. The only way to determine herd immunity is by performing antibody testing.
To Determine Possible Immunity. Even though the CDC and other public health organizations warn against using antibody test results to make health-related decisions until further studies are completed, antibody tests can still provide valuable information to individuals and organizations about risk factors. We encourage individuals who receive the results of an antibody test to keep those records because it is possible, even likely, that government agencies will eventually enact public policies related to returning to work, school, and other activities based upon antibody test results (issuing so-called “immunity cards”) if studies confirm strong and long-lasting immunity. But at this time the CDC advises against using antibody test results to make decisions about grouping persons residing in or being admitted to congregate settings, such as schools, dormitories, or correctional facilities, or to make decisions about returning persons to the workplace.
How to Perform Antibody Testing
To maximize antibody test performance and make stronger conclusions about test results, the CDC recommends three key strategies:
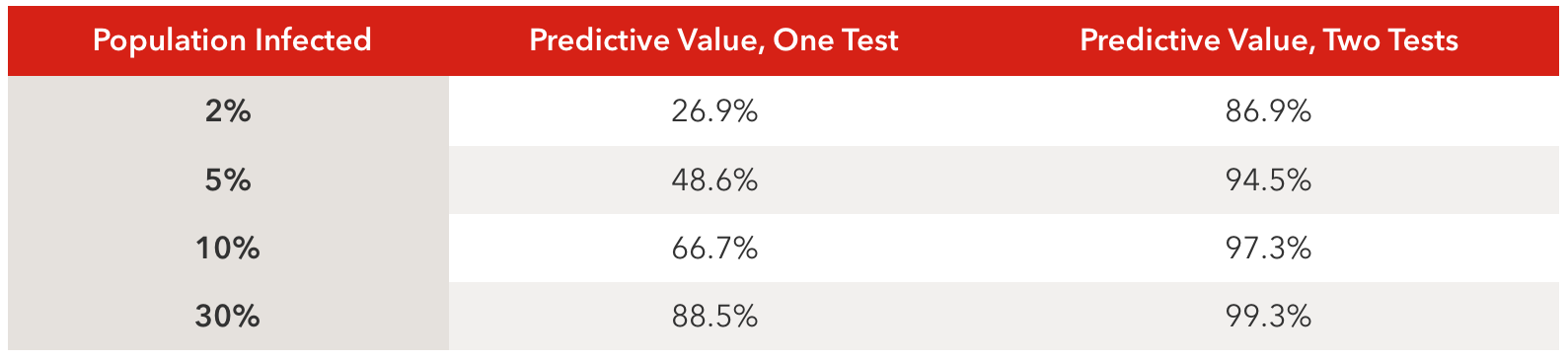
Choose a test with a high specificity (over 95% if possible) to increase the odds the test is correct, especially in populations where the spread of the virus is less than 5%.
Focus on testing people with a high probability of having coronavirus antibodies, such as persons with a history of COVID-19-like illness.
Use two different antibody tests (called orthogonal testing) to increase the predictive value by 10-60%, depending on the prevalence of the virus in a population.
Employing these strategies will increase the predictive value of the tests, meaning the test result will be more likely to be accurate. The best thing a lab or physician can do to increase the accuracy of antibody testing is to use two different tests with high sensitivity and specificity together. Below is a table showing predictive values of using one versus two tests.
Table values assume tests with 90% sensitivity and 95% specificity.
Our antibody tests from Autobio and Healgen are among the best in the world and far exceed the CDC’s recommended 90% sensitivity and 95% specificity thresholds for high-performance testing:
Autobio Antibody Test
Sensitivity: 99.0%
Specificity: 99.0%
Healgen Antibody Test
Sensitivity: 100.0%
Specificity: 97.5%
Used together, our tests return a positive predictive value of 99.5% and a negative predictive value of 99.9%. This means if both tests give a positive result, there is a 99.5% probability it is the correct result. If both tests give a negative result, there is a 99.9% probability it is the correct result. Bear in mind that testing must be performed after a person has developed antibodies, which, on average, takes 13 days. One hundred percent development has been observed by Day 19 according to recent studies.
Administering our antibody tests requires no special skill, training, or equipment. They work much the same as a pregnancy test and use the same basic technology — a lateral flow assay that detects antibodies.
Antibody Testing Procedure
Step 1
Add blood drops and buffer.
Step 2
Wait 10 minutes.
Step 3.
Read results.
Test Cost, Medicare and Insurance Reimbursement
The CMS (Center for Medicare and Medicaid Services) reimbursement rate for our tests is $45.23 per test. Private health insurance companies are mandated by the CARES Act to fully cover antibody testing and are typically reimbursing at rates up to 125% or higher than Medicare depending on the company and test administered. Many private health insurance companies only reimburse FDA EUA authorized tests.
We have two FDA EUA authorized antibody tests available from Autobio and Healgen.
Using the CDC’s recommended dual testing method for each patient (orthogonal testing) to improve accuracy, the table below provides a rough calculation of gross profit before specimen collection, transport, processing, and staff costs. No special equipment is required to perform antibody tests and results are obtained in 15 minutes.
Conclusion
We hope to have provided you with some valuable information about coronavirus antibodies, immunity, maximizing test performance, and financials. If you have any questions, please do not hesitate to contact us about testing or ordering antibody kits.
*The information in this document is based upon our interpretation of the best information available to us. It is not intended to serve as an authoritative source for COVID-19 or testing. The science and understanding of coronavirus, as well as reimbursement rates and pricing is subject to change at any time. Providers are responsible for independently verifying and weighing other sources of information prior to making decisions about testing. The pricing provided in this article is subject to change any time and may not be updated in a timely manner. 6/11/2020.