FDA adds expiration date information to rapid test listings
On April 29, 2022, the U.S. Food and Drug Administration updated its list of Emergency Use Authorizations (EUA) of rapid tests to include expiration date extension letters.
On April 29, 2022, the U.S. Food and Drug Administration updated its list of Emergency Use Authorizations (EUA) of rapid tests to include expiration date extension letters.
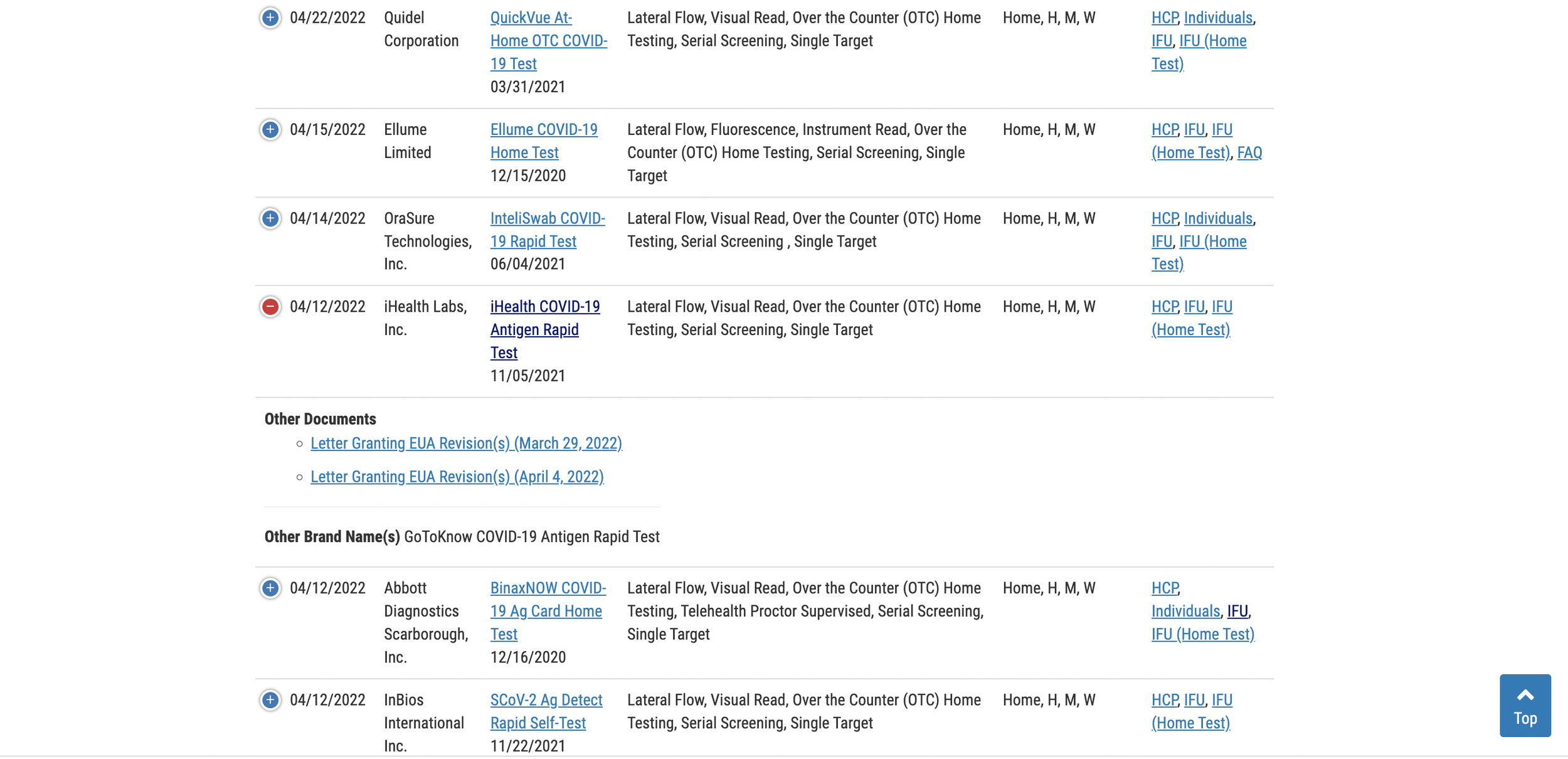
The updated table for rapid antigen tests can be viewed here.
Scroll down the list to locate your test, then click the (+) to the left of the test name to expand the row and access the expiration date extension documents. If you cannot find your test on the first page, just toggle the list menu to show all listings. By default, the page only displays 25 tests.
Stability of Rapid Tests
Most lateral flow immunoassay test cassettes, like those used in Covid-19 rapid antigen and rapid antibody tests, remain stable for years under normal storage conditions. However, the FDA only allows the manufacturer to print an expiration date on the box that is supported by real-time stability data. Since Covid is a new disease and all tests have been recently developed, most tests will only initially be granted a short 3, 6, or 9 month expiration date.
As manufacturers continue submitting new stability data to the FDA on a rolling basis the tests are granted extensions beyond those printed on the box at time of manufacture. So even if the expiration date printed on your box is coming up soon, or has passed, there’s a very good chance your tests haven’t really expired. Now you can cross-reference the FDA website to see if any extensions were granted. Most Covid rapid tests have not expired yet and will ultimately be granted a final shelf-life of 24 to 36 months.
California Department of Public Health endorses use of rapid tests past FDA expiration dates
The California Department of Public Health went even further than the FDA on March 4, 2022, and endorsed the use of over-the-counter rapid tests even beyond the FDA authorized expiration, indicating that as long as the control lines register properly on the test, it can be used. The extension is valid until further notice.
Here is the CDPH’s full public notice, the image is linked to the original statement posted on its website.
If you have any questions about expiration dates, or would like us to help you find your test’s expiration date with the extensions, just contact us.