Specifications
CLIA Waived
Test type: Rapid Antibody
Run time: 10 minutes
Collection type: Finger-prick capillary, or whole blood, plasma, or serum
Covid-19 Antibodies: 100.0% PPA, 99.2% NPA
CPT Codes: 86328
Manufacturer: AssureTech
Storage & Handling
Finger-prick capillary blood may be taken and used at point-of-care.
Cap and store the serum or plasma samples at 2-8°C for no more than 24 hours prior to testing.
For long-term storage, freeze the serum or plasma samples at -20°C. Avoid multiple freeze-thaw cycles.
Mix thawed samples thoroughly by low speed vortexing or by inverting 10 times. Bring samples to room temperature prior to testing for at least 30 minutes. Visually inspect the samples. If layering or stratification is observed, continue mixing until samples are visibly homogeneous.
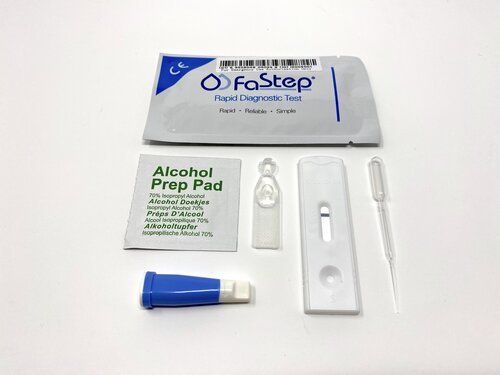
Box Components
Test cassettes (20)
Lancets (20)
Blood collection pipets (20)
Reagent buffer capsules (20)
Instructions for Use (1)
Manuals & Documents
Intended Use
The Assure COVID-19 IgG/IgM Rapid Test Device is a rapid lateral flow chromatographic immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human venous whole blood (sodium EDTA), serum, plasma (sodium EDTA) and fingerstick whole blood. The Assure COVID-19 IgG/IgM Rapid Test Device is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The Assure COVID-19 IgG/IgM Rapid Test Device should not be used to diagnose acute SARS-CoV-2 infection. Use of this test with all authorized specimen types is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet requirements to perform waived, moderate or high complexity tests.